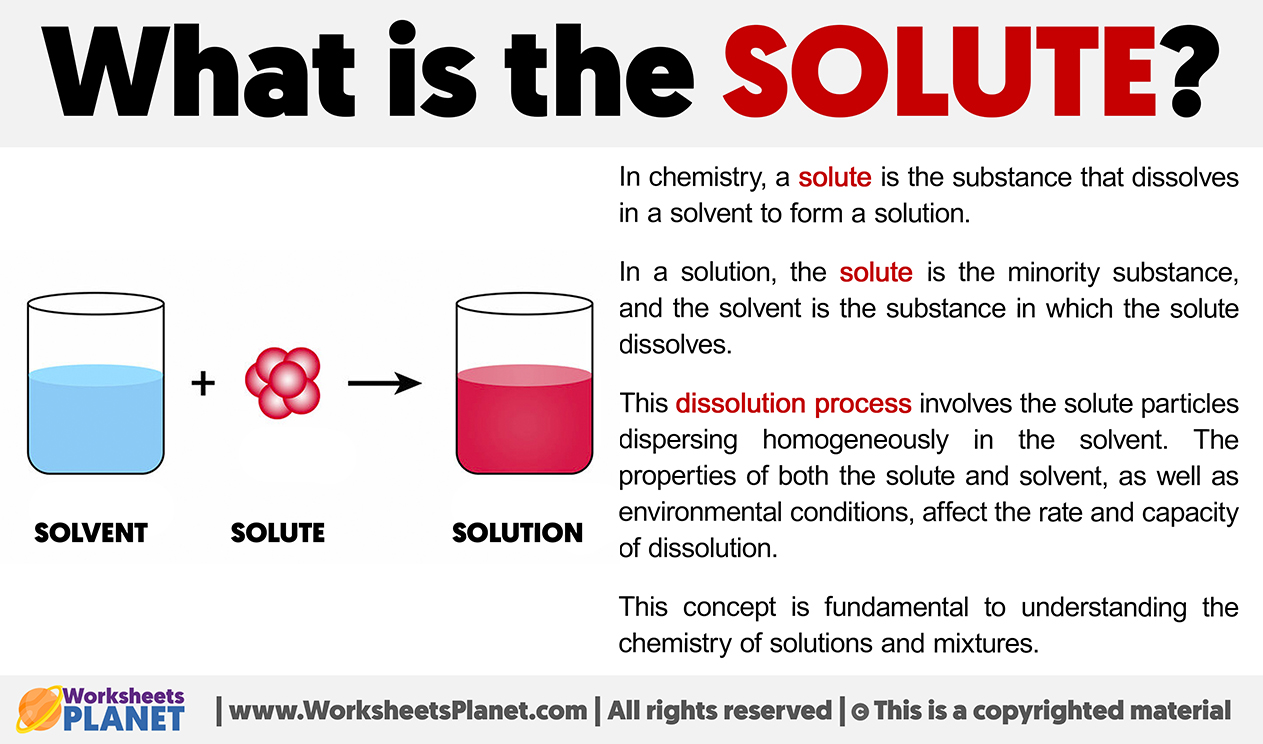
In chemistry, a solute is the substance that dissolves in a solvent to form a solution. In a solution, the solute is the minority substance, and the solvent is the substance in which the solute dissolves.
This dissolution process involves the solute particles dispersing homogeneously in the solvent. The properties of both the solute and solvent, as well as environmental conditions, affect the rate and capacity of dissolution.
This concept is fundamental to understanding the chemistry of solutions and mixtures.