Metal + Non-metal atoms form ionic bonds. Metal atoms give up electrons forming cations, and non-metal atoms accept them forming anions”.Remember that a negative and a positive electrical charge attract each other.
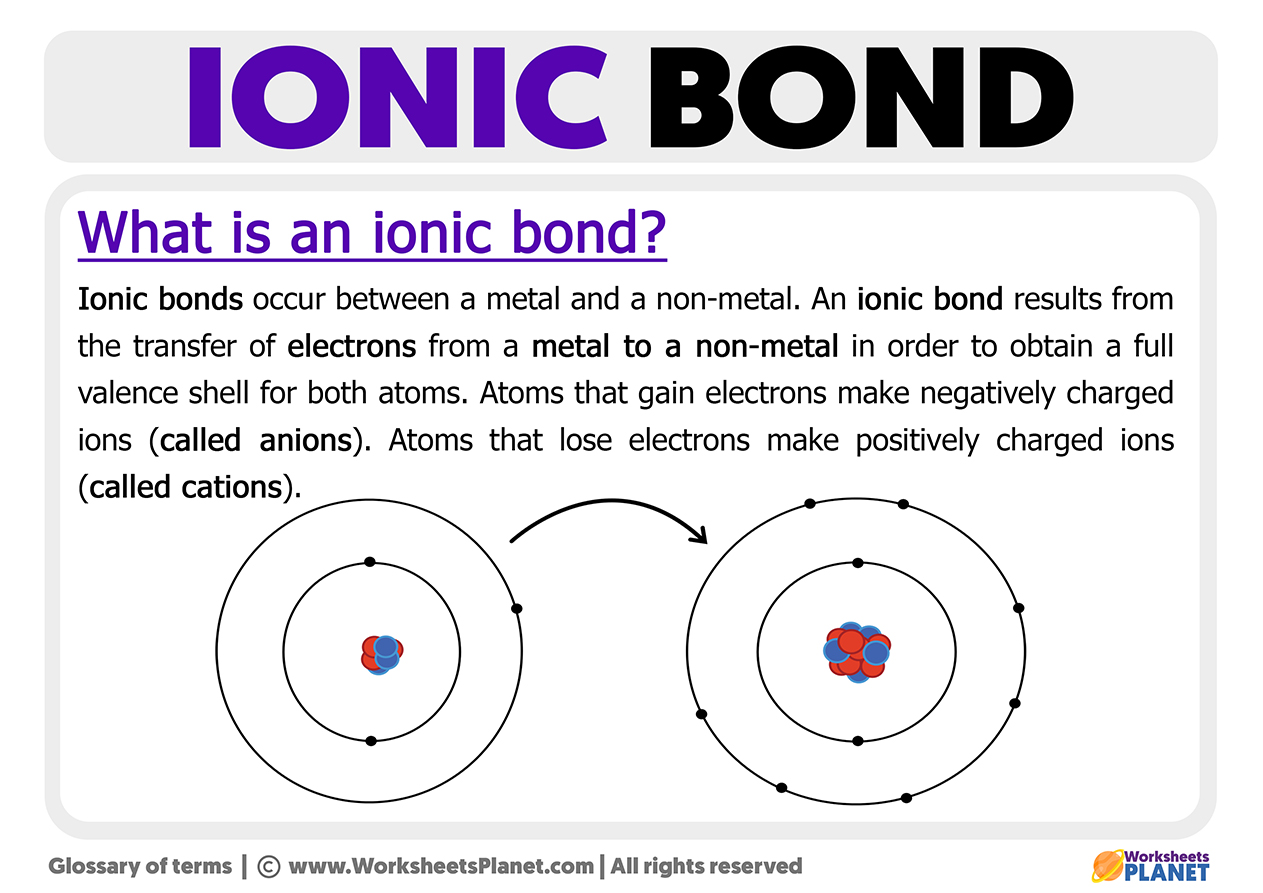
What will happen to the cation (positive charge) and anion (negative charge) formed by the two atoms meeting?
Well, they attract each other and form an ionic bond. Once the bond is made, it does not form true molecules. The atoms are only held together by electrical forces. The result is positive and negative ions that attract each other, called ionic bonds, but not true molecules.

