An endothermic reaction absorbs energy, in the form of heat or radiation, from its surroundings. The absorption of energy or heat is what all endothermic reactions have in common; their nature and the transformations involved are very diverse. How much heat must they absorb? The answer depends on its thermodynamics: the temperature at which the reaction occurs spontaneously.
Example of Endothermic Reaction
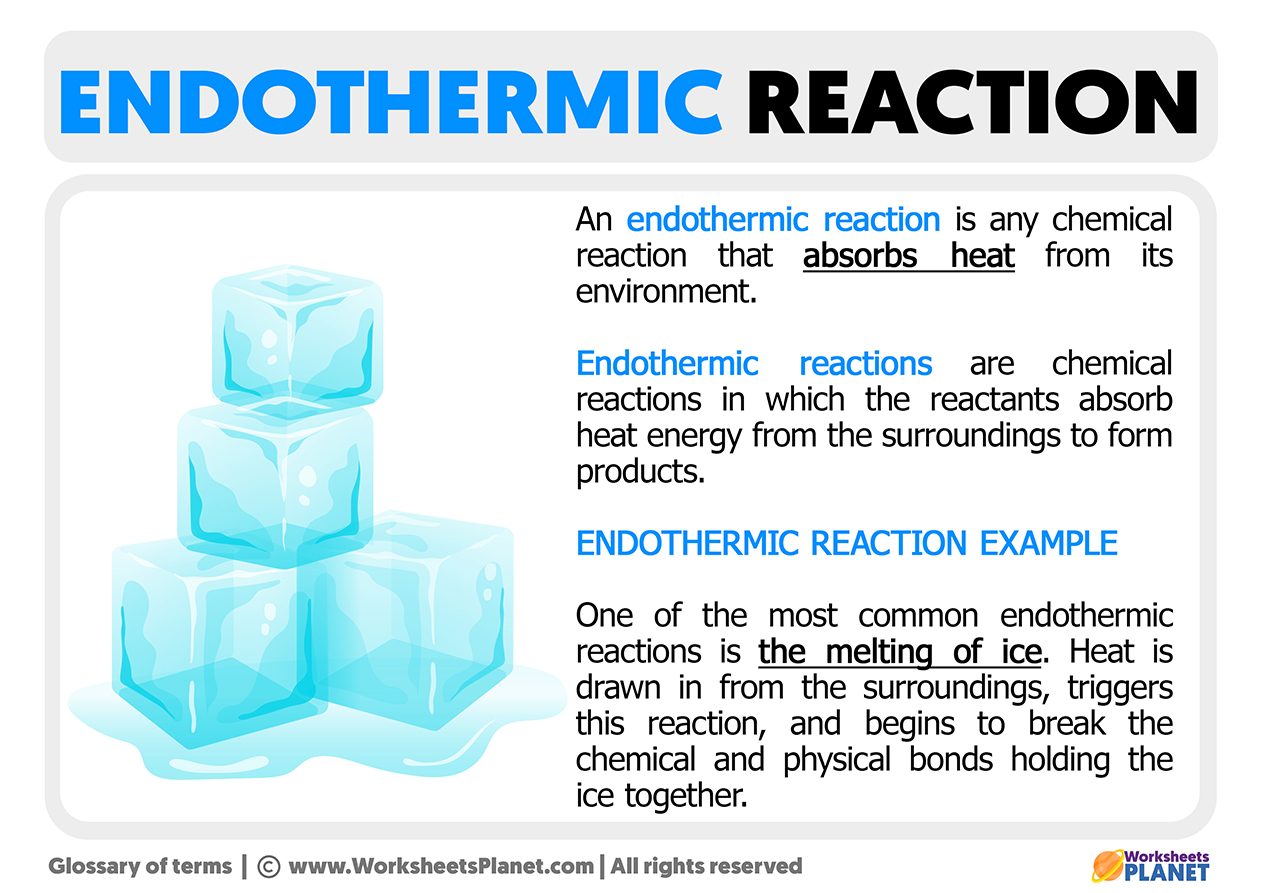
One of the most iconic endothermic reactions is the change of state from ice to liquid water. Ice needs to absorb heat until its temperature reaches approximately 0ºC. At that temperature, its fusion becomes spontaneous, and the ice will absorb until it has completely melted.
Characteristics of an endothermic reaction
- The main characteristic of an endothermic reaction is that the products are more energetic than the reactants.
- The difference of energy between them (ΔH) is always positive (HProduct-HReactive > 0). This characteristic implies that heat or energy from the surroundings must be absorbed to supply this energy need.
- When the bonds formed do not provide stability comparable to the amount of energy required to break the old bonds, an endothermic reaction occurs. This is why additional energy is needed to promote the breaking of the more stable bonds in the reactants.
- Although it does not apply to all endothermic reactions, several of them cause a drop in the temperature of their surroundings. This is because the absorbed heat comes from somewhere. Consequently, if the conversion of A and B were to take place inside a container, it would cool down.
- The more endothermic the reaction, the colder the container and its surroundings will become. Some reactions can even form a thin shell of ice as if they had just come out of a refrigerator.

