A subatomic particle is a particle that is even smaller in size than an atom. It can be found in elemental or compound forms when it is made up of other subatomic particles. As we know, subatomic particles are divided into three cathegories, and electrons formed one of them.
Definition of Electron
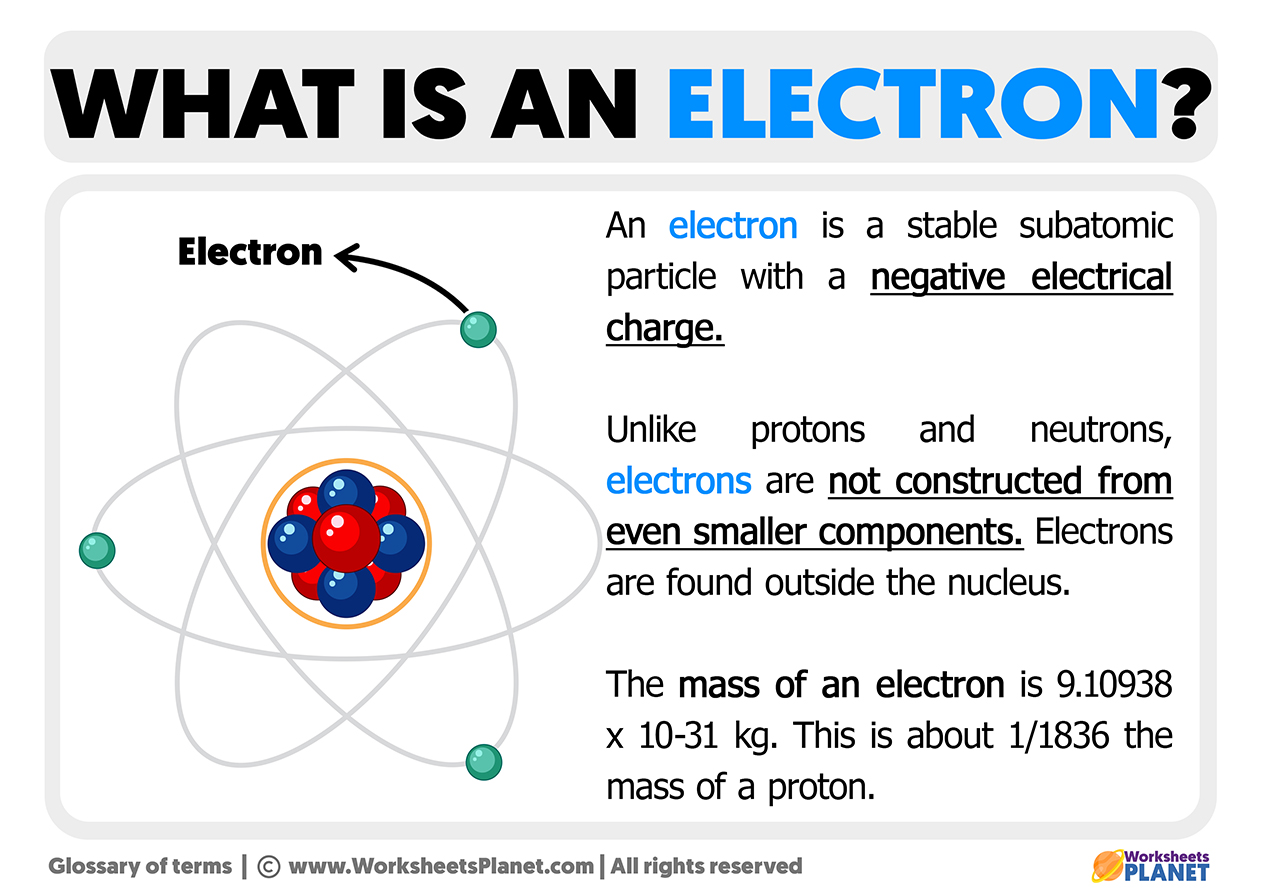
An electron is a negatively charged subatomic particle. It may be free and not attached to any atom, or it may be attached to the nucleus of an atom. An electron is also generally defined as an elementary particle.
So, what are electrons?
Electrons are subatomic elements and the lightest essential particles that make up an atom with the least negative electrical charge and are located around the nucleus.
Characteristics of electrons
The main characteristics of electrons are the following ones:
- The symbol e- represents them.
- They are subatomic particles that have a negative elementary electrical charge.
- An electron does not present any type of known components or substructure, which is why it is defined as an elementary particle.
- It has a mass of about 1836 times less than the mass of the proton.
- It has an intrinsic angular momentum or spin, which has a half-integer value in units of ħ, which means that it is a fermion.
- They are much smaller in size than other atomic particles.
- They emit or absorb a photon when they move from one energy level to another.

