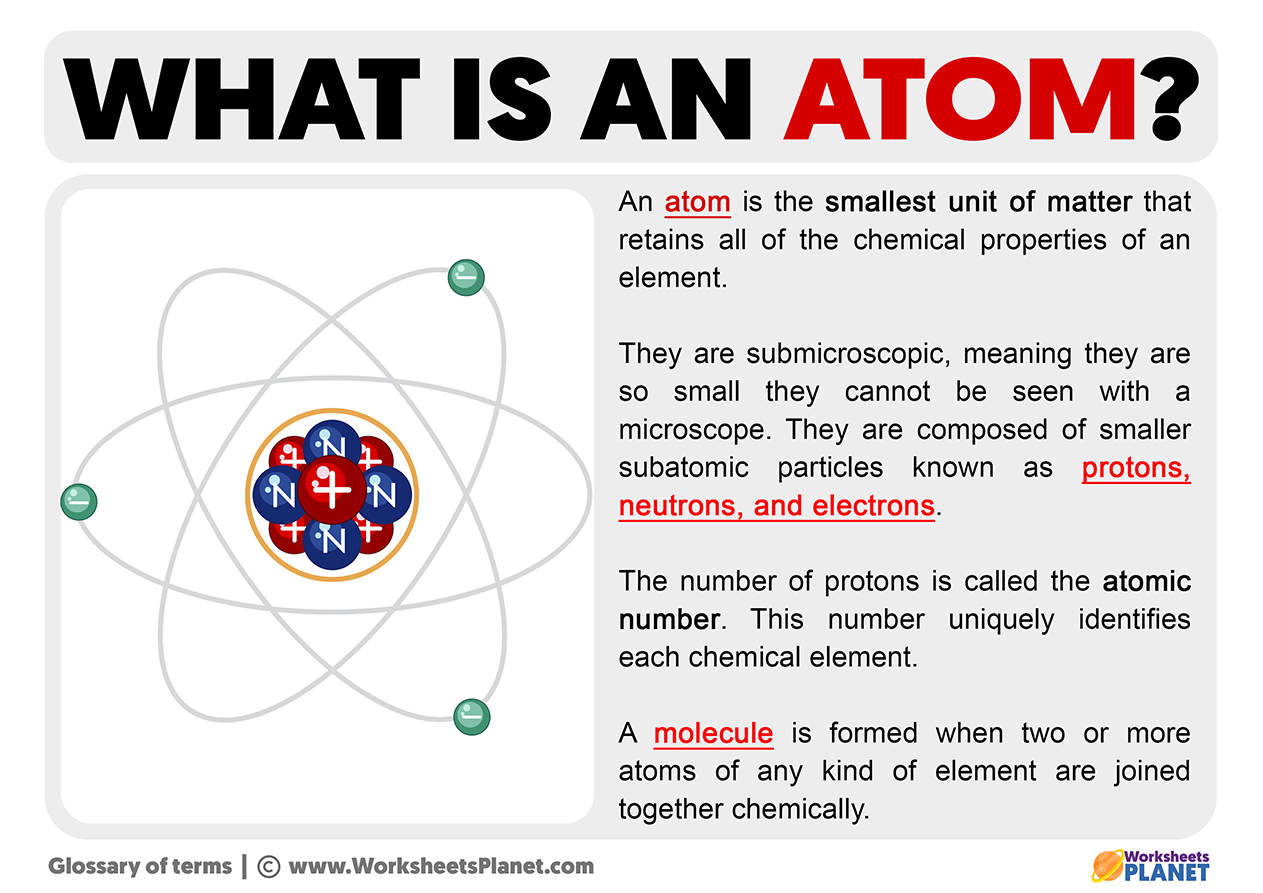
The atom is the name of the smallest unit of matter. Atoms organize all kinds of matter; you can find them in nature, the physical world, and everything you can see and touch. The atom is the smallest particle into which an element can be divided. An atom is made up of three substructures: neutrons, protons, and electrons.
An atom is made up of three substructures: electrons (negative charge), neutrons (neutral charge), and protons (positive charge). An atom is divided into two parts. The nucleus contains the neutrons, protons, and the crust, which only contains electrons. A molecule is formed when two or more atoms of any kind of element are joined together chemically.
Parts of an Atom
Electrons, neutrons, and protons are subatomic particles that make up the structure of the atom. The lightest are electrons, which weigh 1,836 times less than protons. Neutrons have no electrical charge, and their weight is very similar to that of protons.
Protons and neutrons are in the atom’s nucleus, which is why they are also called nucleons. It is nuclear energy that makes neutrons and protons stick together. Surrounding the nucleus is a certain number of negatively charged electrons.
Characteristics of an atom
In chemistry, the basic units are atoms. When a chemical reaction occurs, it is preserved with its original properties. In no case can an atom be destroyed or created. They organize themselves differently, creating a series of links between them.
Depending on their composition, the different chemical elements are represented and distinguished in the periodic table. Each of the elements has an atomic number and a mass number.

