Non-Metal atoms form covalent bonds. Remembering the bond concept (atoms that share electrons and therefore are bonded) The elements on the far right of the periodic table are the non-metallic elements (yellow color). Only these can form covalent bonds.
Non-metallic atoms usually have many electrons spinning in their last orbit (called valence electrons). They tend to gain (take) electrons from another atom instead of giving them up. The problem is that since the two atoms are non-metals, neither will want to give up their electrons to the other.
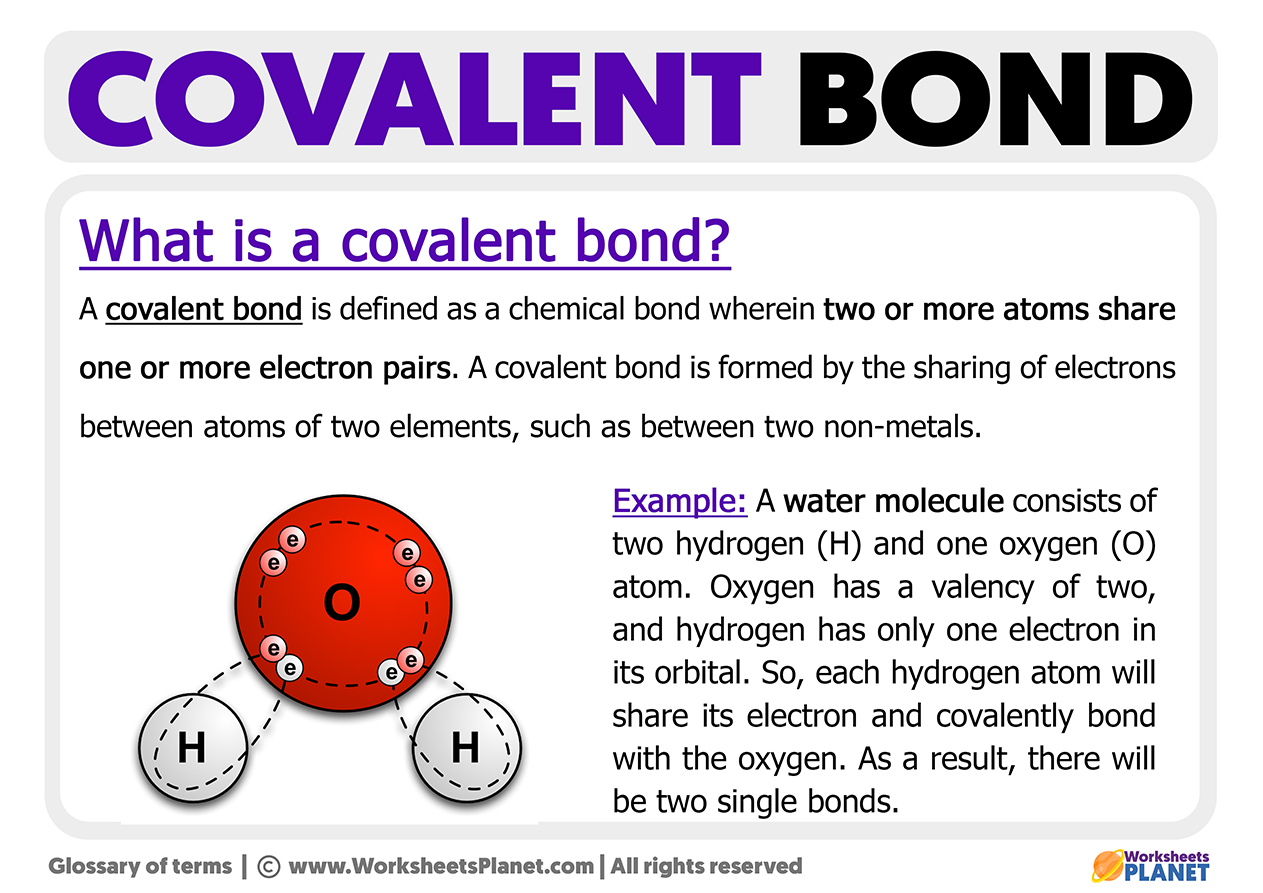
Implications of a Covalent Bond
As non-metal atoms do not want to get rid of electrons, when they meet or join, they share electrons from their last layer instead of giving up or gaining electrons (which would be the case of ionic bonds).
Conclusion: non-metallic atoms cannot give or gain electrons from each other but can share them. Covalent bonds are formed between two Non-Metals, sharing valence electrons.
When non-metal atoms come together, shared electrons will join together, becoming part of the two atoms, thus forming a molecule (several atoms together). Once joined, the two atoms acquire the noble gas structure with eight atoms.
They can be linked by single, double, or triple bonds, depending on the number of electrons they share in the bond.

