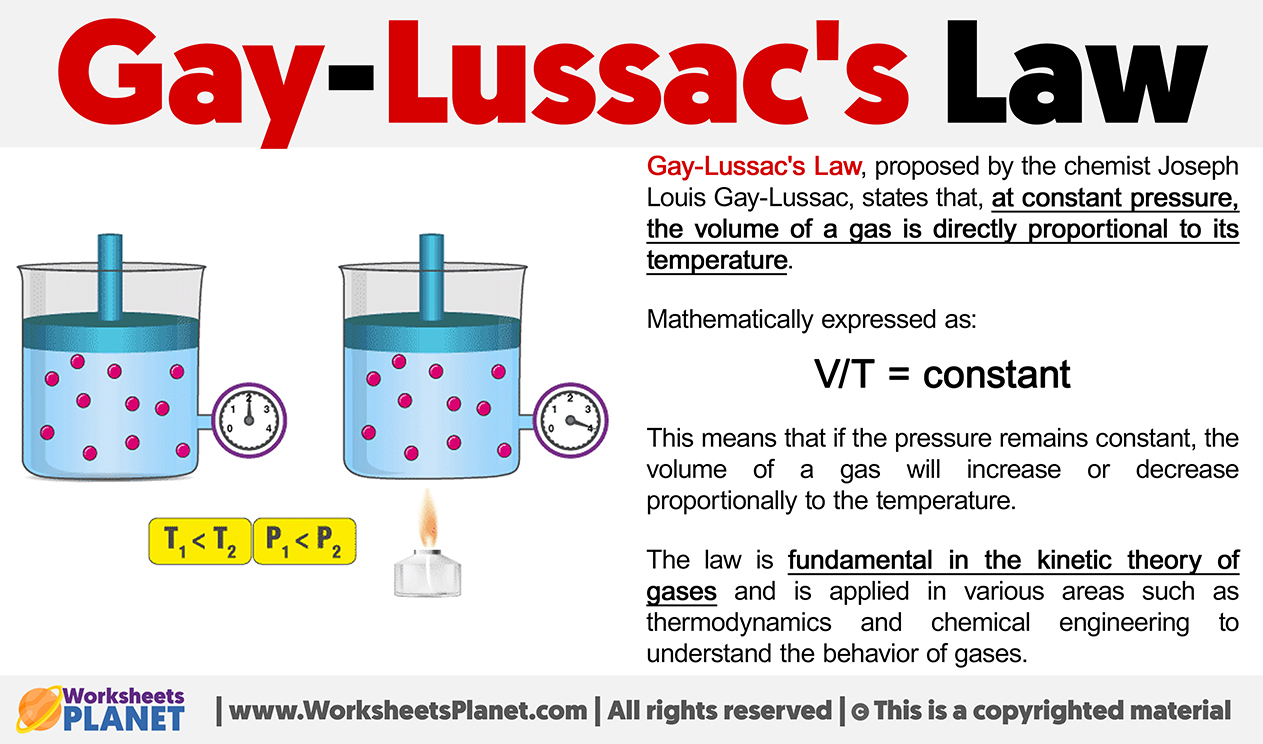
Gay-Lussac’s Law, proposed by the chemist Joseph Louis Gay-Lussac, states that, at constant pressure, the volume of a gas is directly proportional to its temperature.
Mathematically expressed as:
V/T = constant
This means that if the pressure remains constant, the volume of a gas will increase or decrease proportionally to the temperature. The law is fundamental in the kinetic theory of gases and is applied in various areas such as thermodynamics and chemical engineering to understand the behavior of gases.