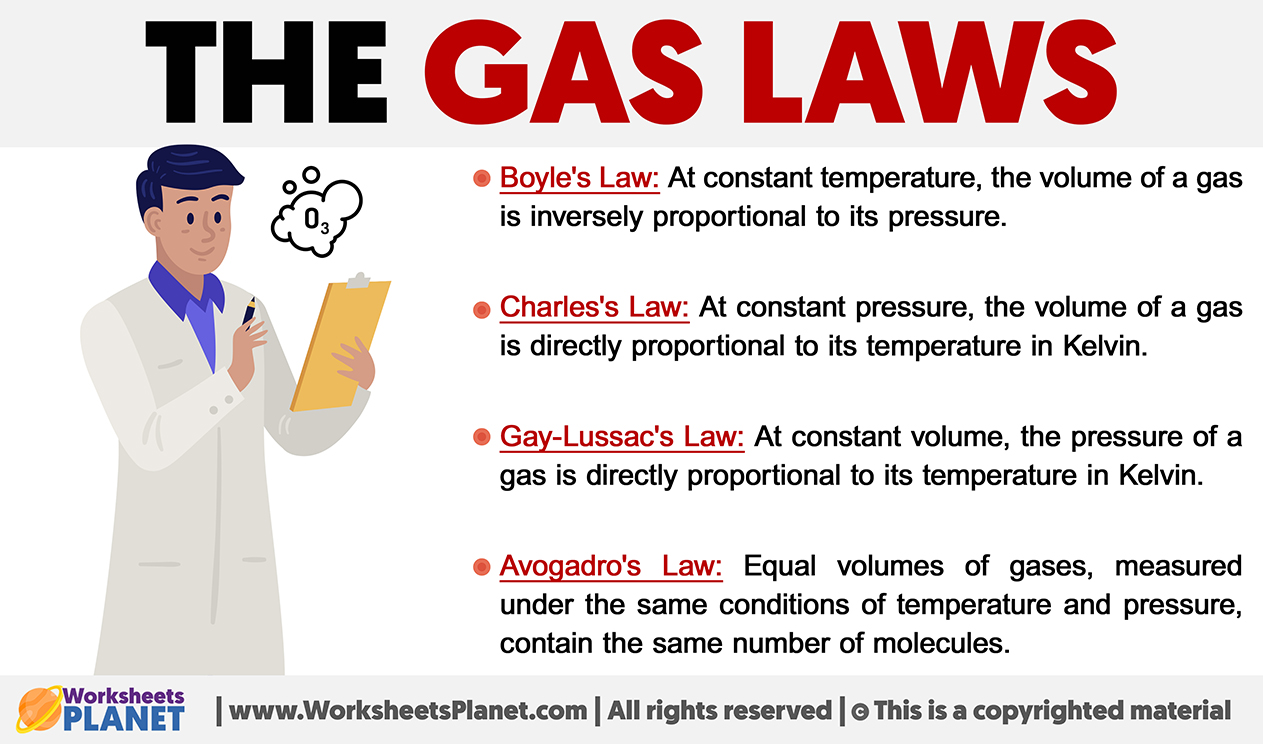
Boyle’s Law: At constant temperature, the volume of a gas is inversely proportional to its pressure.
Charles’s Law: At constant pressure, the volume of a gas is directly proportional to its temperature in Kelvin.
Gay-Lussac’s Law: At constant volume, the pressure of a gas is directly proportional to its temperature in Kelvin.
Avogadro’s Law: Equal volumes of gases, measured under the same conditions of temperature and pressure, contain the same number of molecules.