A reduction reaction involves the gain of electrons by a substance, leading to a decrease in its oxidation state. Typically paired with oxidation reactions in redox processes, reduction reactions play a key role in chemical transformations.
They occur in diverse contexts, such as metal extraction, battery operation, and biological processes like cellular respiration, influencing the behavior and properties of substances.
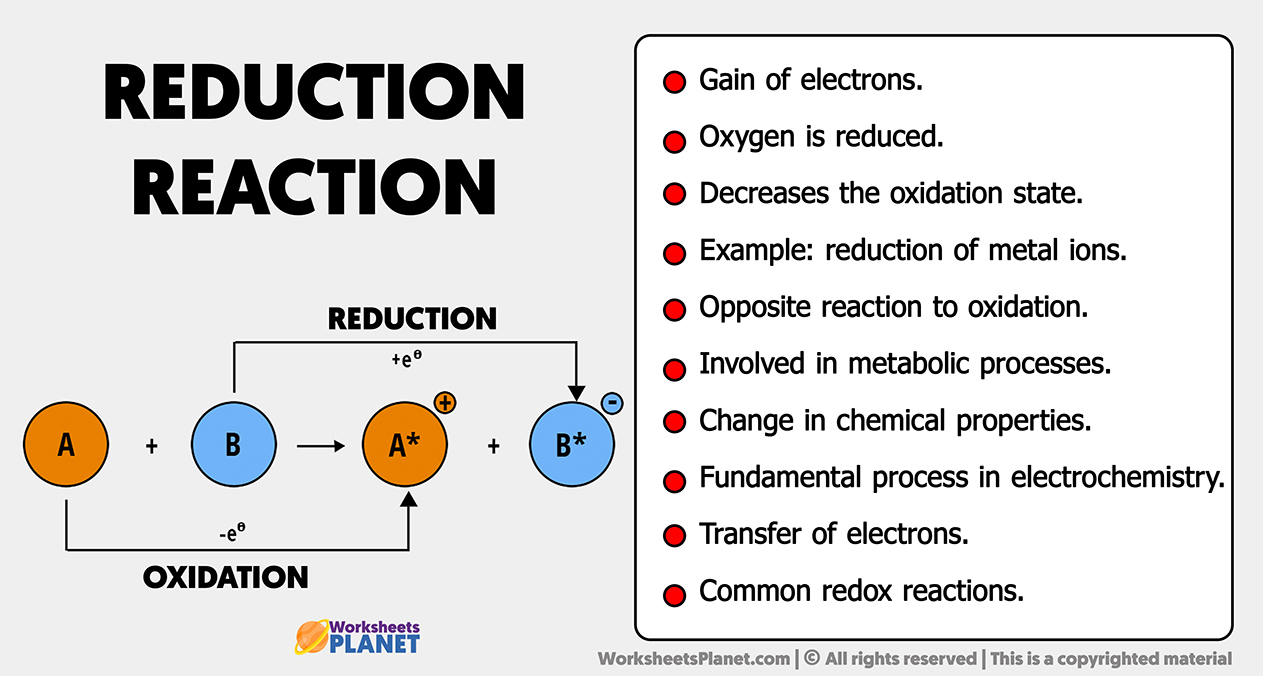
- Gain of electrons.
- Oxygen is reduced.
- Decreases the oxidation state.
- Example: reduction of metal ions.
- Opposite reaction to oxidation.
- Involved in metabolic processes.
- Change in chemical properties.
- Fundamental process in electrochemistry.
- Transfer of electrons.
- Common redox reactions.

