A precipitation reaction occurs when two solutions combine, leading to the formation of an insoluble solid, known as a precipitate. This process results from the mixing of ions that react to produce a compound with limited solubility.
Commonly observed in chemical reactions, precipitation reactions play a role in the formation of various minerals, the purification of water, and analytical chemistry techniques.
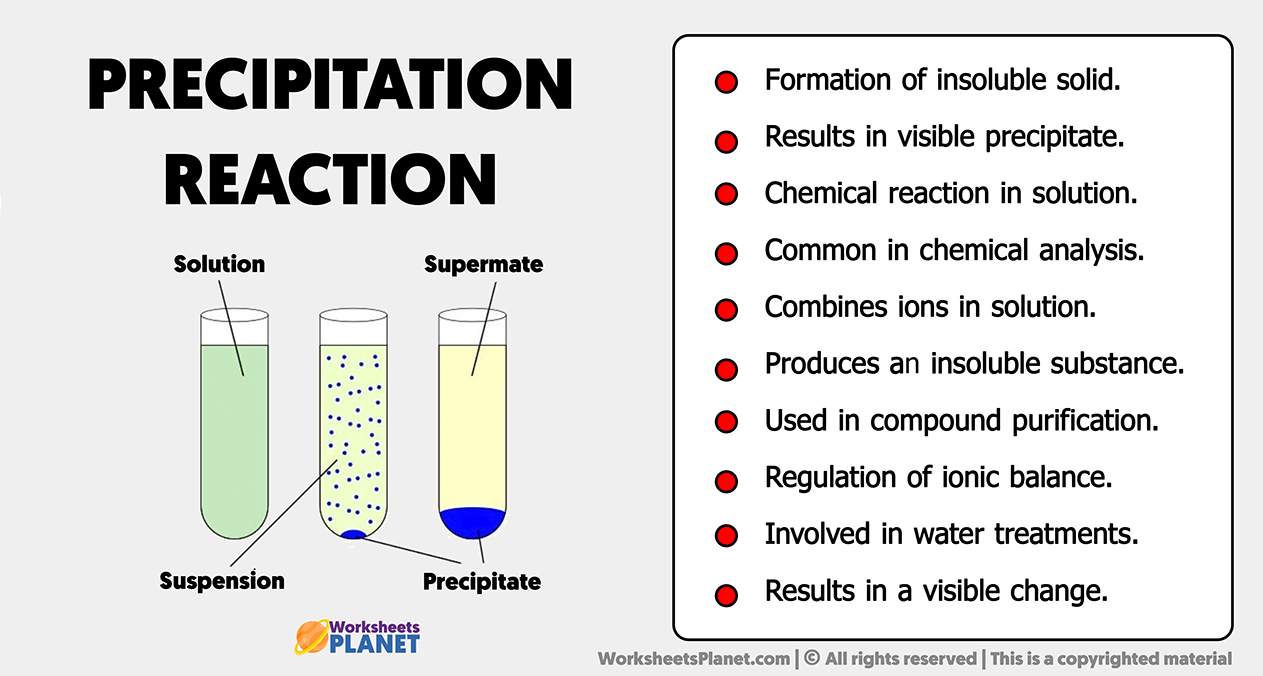
- Formation of insoluble solid.
- Results in visible precipitate.
- Chemical reaction in solution.
- Common in chemical analysis.
- Combines ions in solution.
- Produces an insoluble substance.
- Used in compound purification.
- Regulation of ionic balance.
- Involved in water treatments.
- Results in a visible change.

