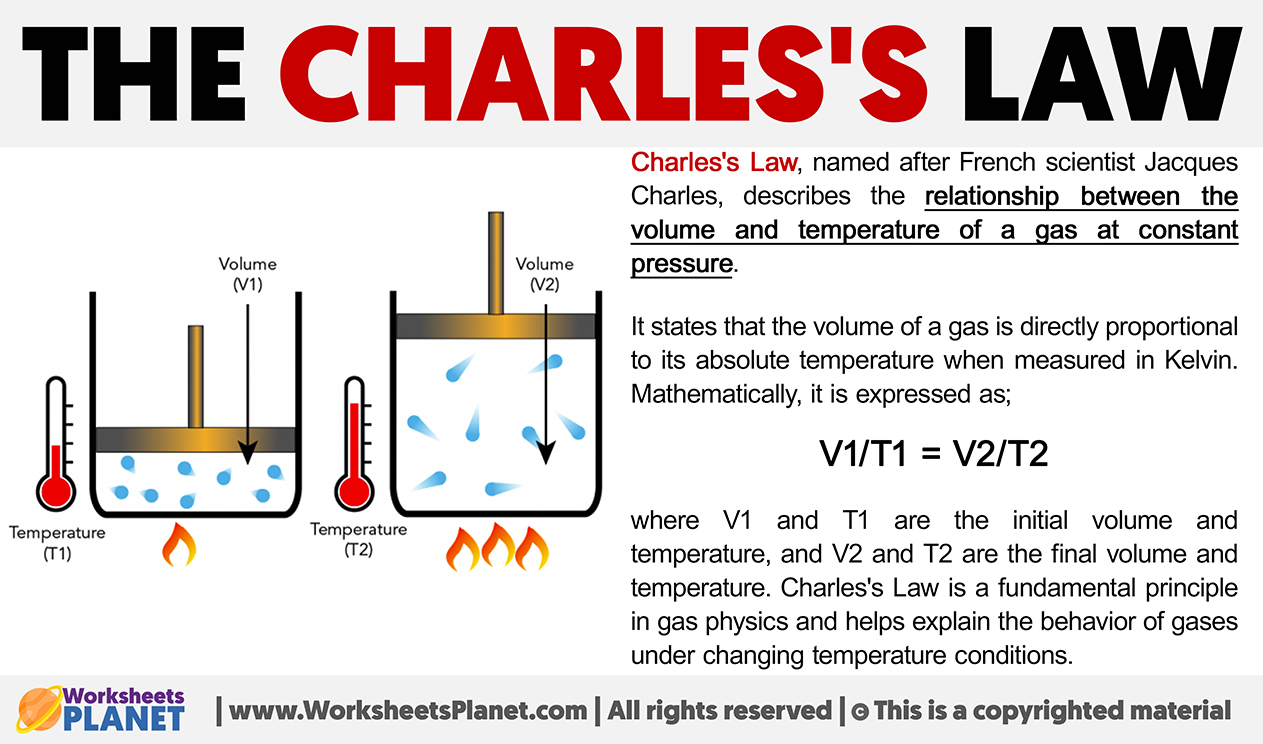
Charles’s Law, named after French scientist Jacques Charles, describes the relationship between the volume and temperature of a gas at constant pressure.
It states that the volume of a gas is directly proportional to its absolute temperature when measured in Kelvin. Mathematically, it is expressed as;
V1/T1 = V2/T2
where V1 and T1 are the initial volume and temperature, and V2 and T2 are the final volume and temperature. Charles’s Law is a fundamental principle in gas physics and helps explain the behavior of gases under changing temperature conditions.