A proton is a positively charged subatomic particle found in the nucleus of an atom. It contributes to the atom’s mass and helps define its identity as an element. Protons, along with neutrons, make up the atomic nucleus, while electrons orbit around them.
The number of protons in an atom determines its atomic number, which distinguishes one element from another.
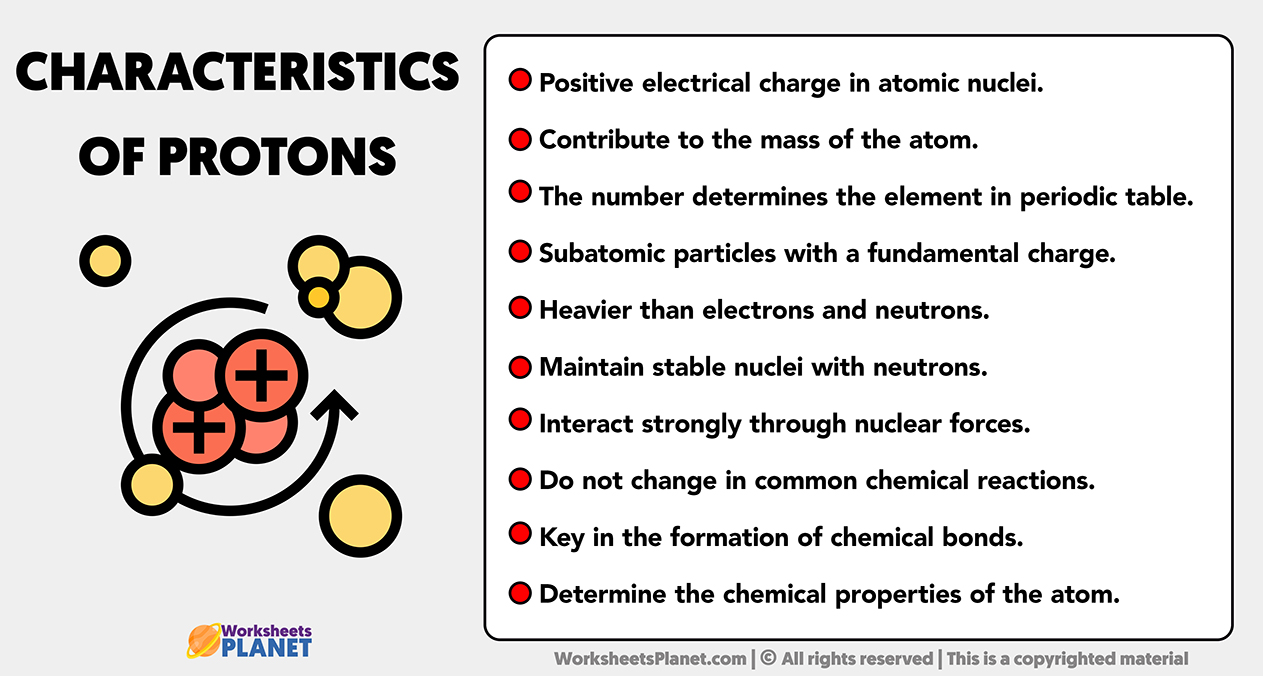
Main characteristics of Protons
- Positive electrical charge in atomic nuclei.
- Contribute to the mass of the atom.
- The number determines the element in periodic table.
- Subatomic particles with a fundamental charge.
- Heavier than electrons and neutrons.
- Maintain stable nuclei with neutrons.
- Interact strongly through nuclear forces.
- Do not change in common chemical reactions.
- Key in the formation of chemical bonds.
- Determine the chemical properties of the atom.

