Nonmetals are elements that lack typical metallic properties. They are generally poor conductors of heat and electricity, lack metallic luster, and are often brittle.
Nonmetals include elements like hydrogen, helium, carbon, nitrogen, oxygen, fluorine, and neon. They tend to form covalent bonds with other nonmetals and exhibit diverse physical states, ranging from gases (like nitrogen) to solids (like sulfur).
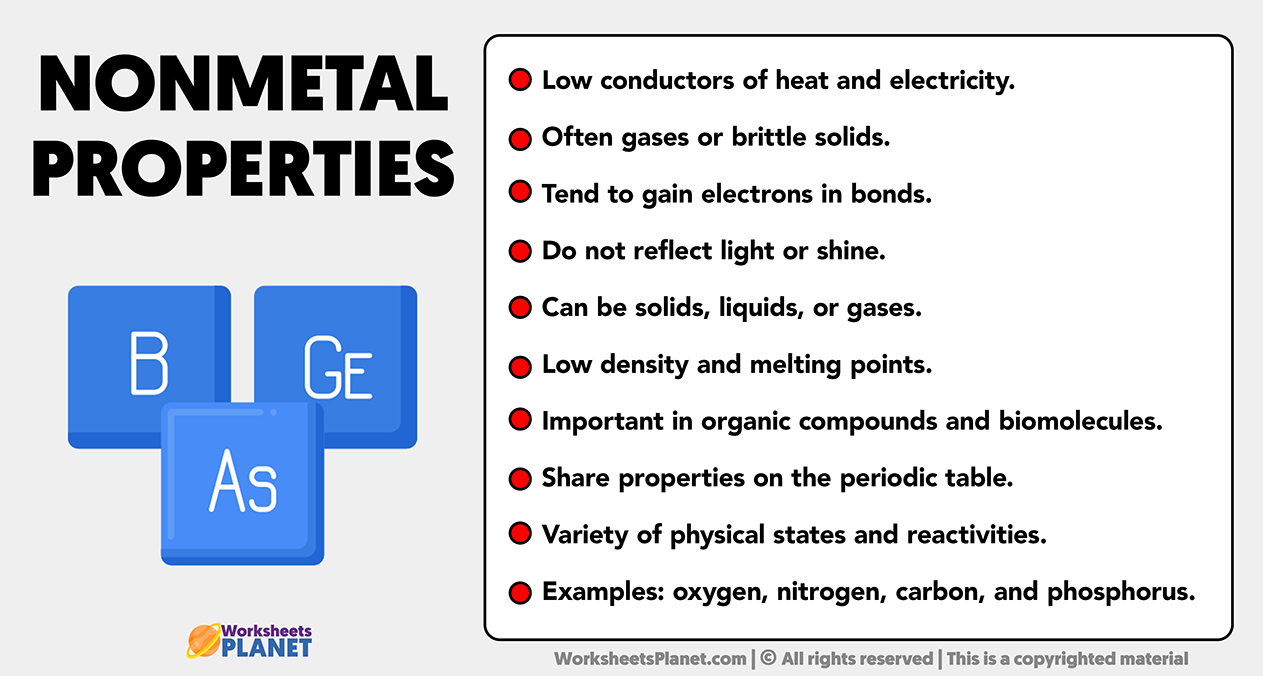
Main Properties of Nonmetal Elements
- Low conductors of heat and electricity.
- Often gases or brittle solids.
- Tend to gain electrons in bonds.
- Do not reflect light or shine.
- Can be solids, liquids, or gases.
- Low density and melting points.
- Important in organic compounds and biomolecules.
- Share properties on the periodic table.
- Variety of physical states and reactivities.
- Examples: oxygen, nitrogen, carbon, and phosphorus.

