The atom is the elementary unit of matter capable of preserving the characteristics of the element to which it belongs, regardless of the chemical transformations that occur in it.
Atoms have a positively charged nucleus of microscopic dimensions and a shell of negatively charged electrons, which move around the nucleus on one or more orbits (n).
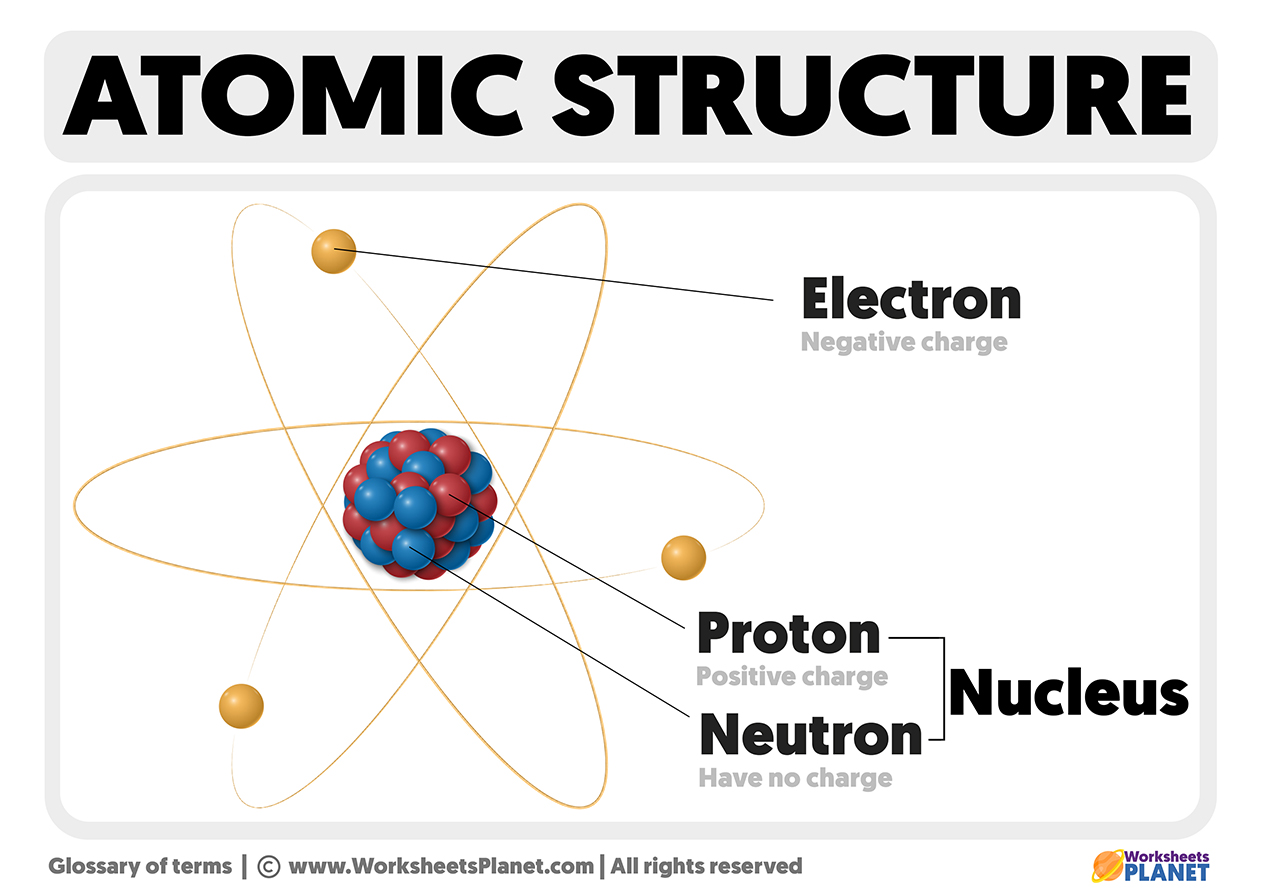
The nucleus is in the central part of the atom. It contains positively charged particles called protons, and non-electrical charge particles, called neutrons. The mass of neutrons and protons, is approximately equal.
The shell is the outer part of the atom. Here are negatively charged particles, called electrons, arranged at different levels, orbiting the nucleus. Atoms are electrically neutral as they have the same number of electrons as proton Thus, we can say the atomic number also coincides with the number of electrons.

