pH refers to the quantitative measure of the acidity or basicity of aqueous or other liquid solutions. On a scale from O to 14, lower pH solutions are strong acids; higher pH solutions are bases. Solutions measuring pH7 are neutral.
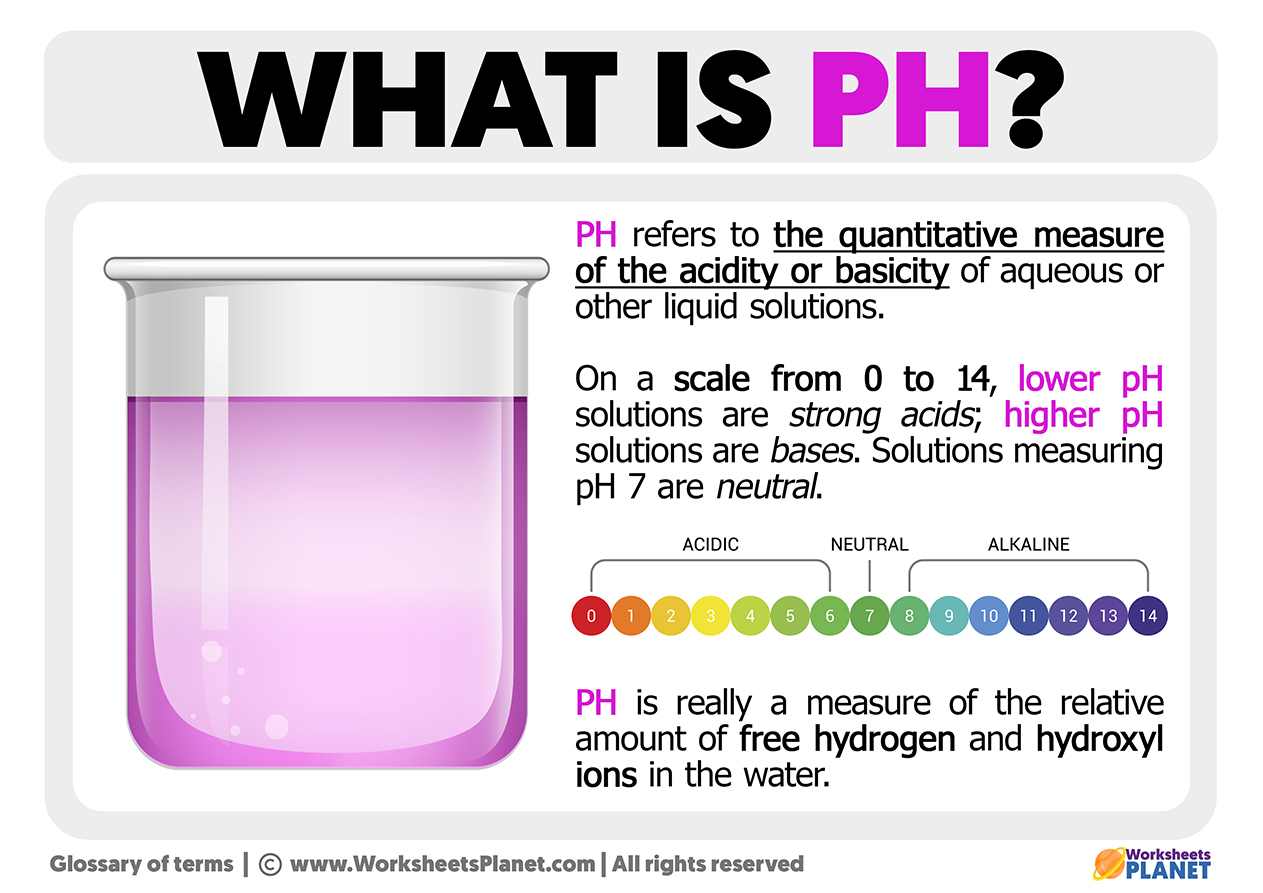
pH is really a measure of the relative amount of free hydrogen and hydroxyl ions in the water.

