An isotope is a variation of the same element with a different number of neutrons in its nucleus. The isotopes of an element have very similar chemical properties because they have the same number of electrons and protons.
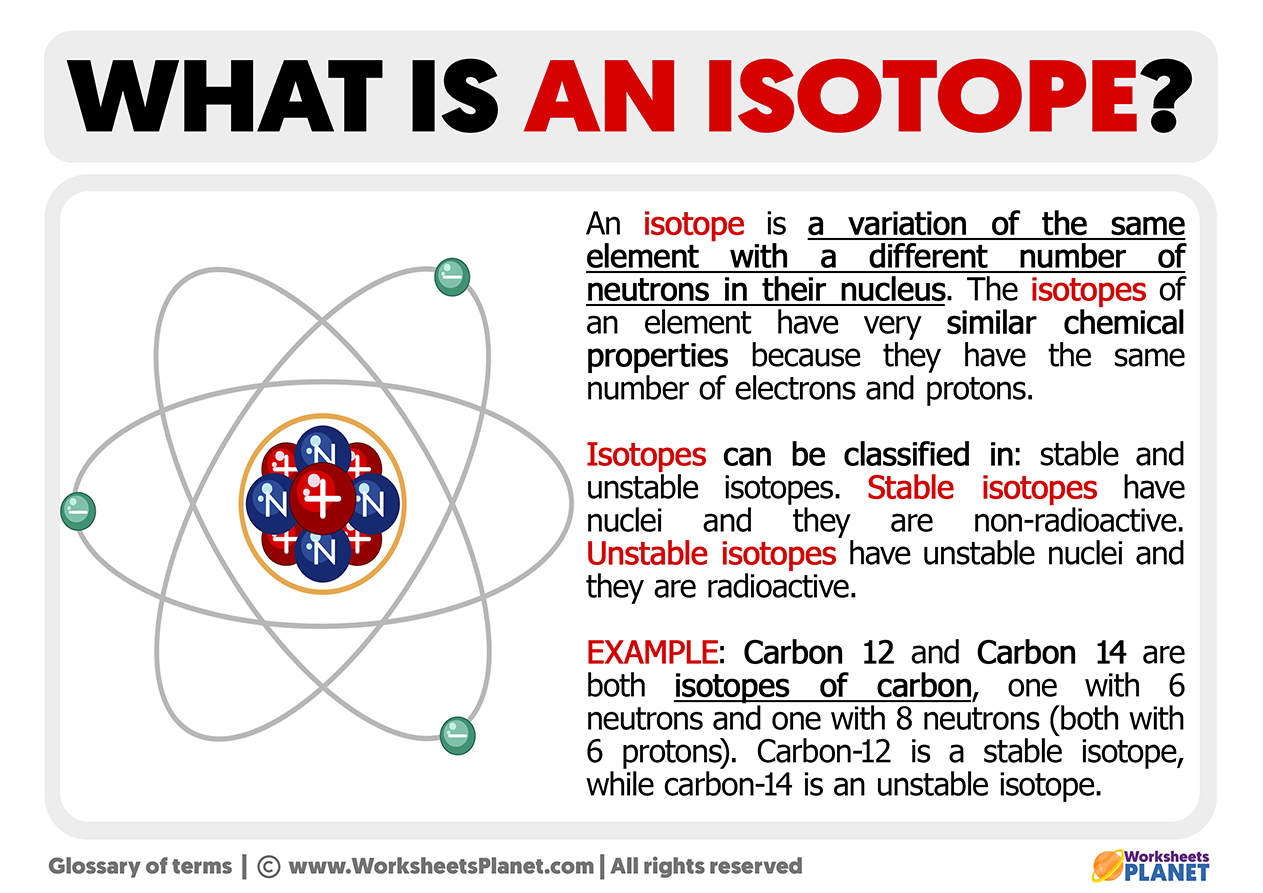
Isotopes can be classified into stable and unstable isotopes. Stable isotopes have nuclei, and they are non-radioactive. Unstable isotopes have unstable nuclei, and they are radioactive.
Example of Isotope: Carbon 12 and Carbon 14 are both isotopes of carbon, one with six neutrons and one with eight neutrons (both with six protons). Carbon-12 is a stable isotope, while carbon-14 is an unstable isotope.

