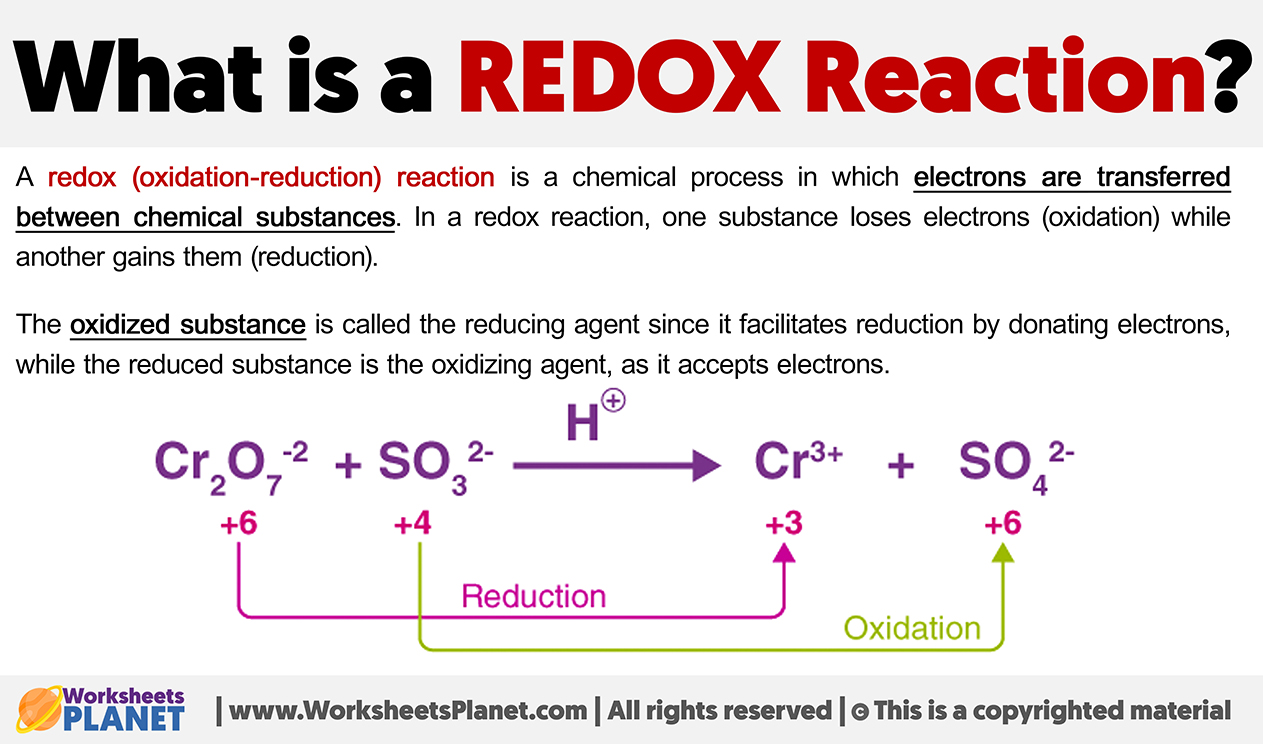
A redox (oxidation-reduction) reaction is a chemical process in which electrons are transferred between chemical substances. In a redox reaction, one substance loses electrons (oxidation) while another gains them (reduction).
The oxidized substance is called the reducing agent since it facilitates reduction by donating electrons, while the reduced substance is the oxidizing agent, as it accepts electrons.