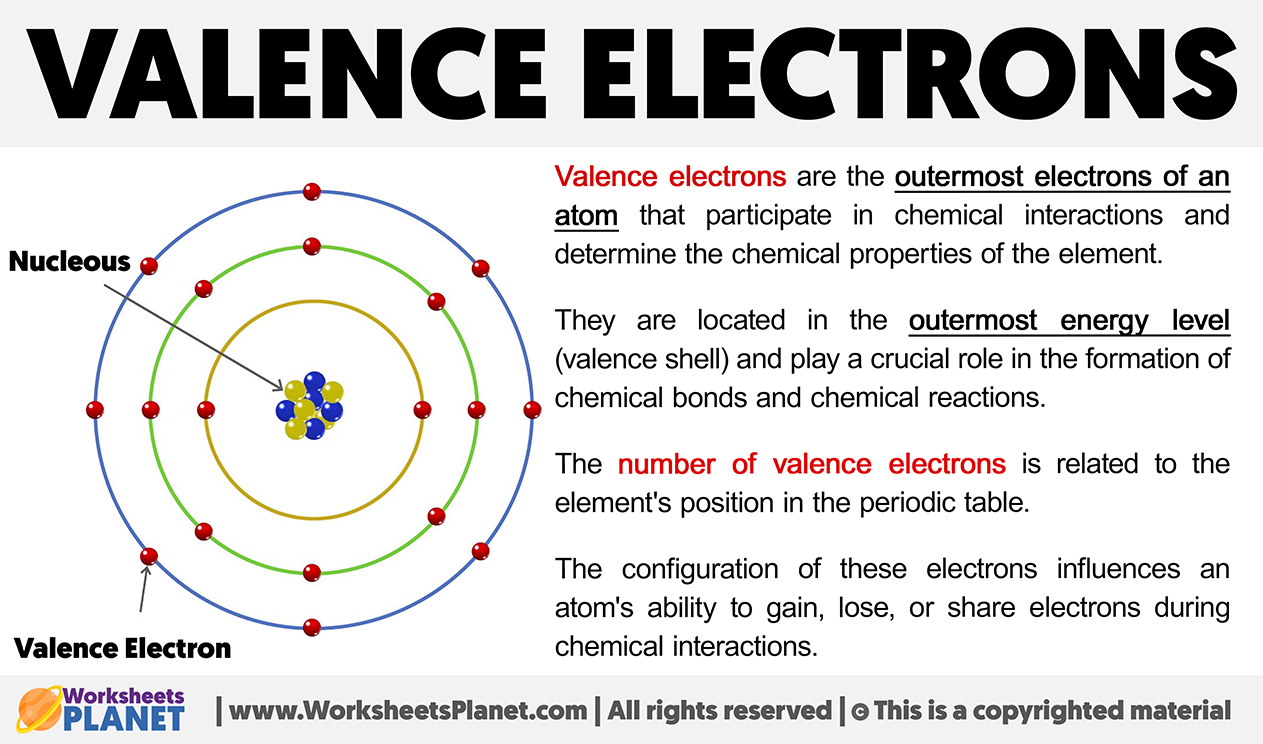
Valence electrons are the outermost electrons of an atom that participate in chemical interactions and determine the chemical properties of the element.
They are located in the outermost energy level (valence shell) and play a crucial role in the formation of chemical bonds and chemical reactions.
The number of valence electrons is related to the element’s position in the periodic table. The configuration of these electrons influences an atom’s ability to gain, lose, or share electrons during chemical interactions.