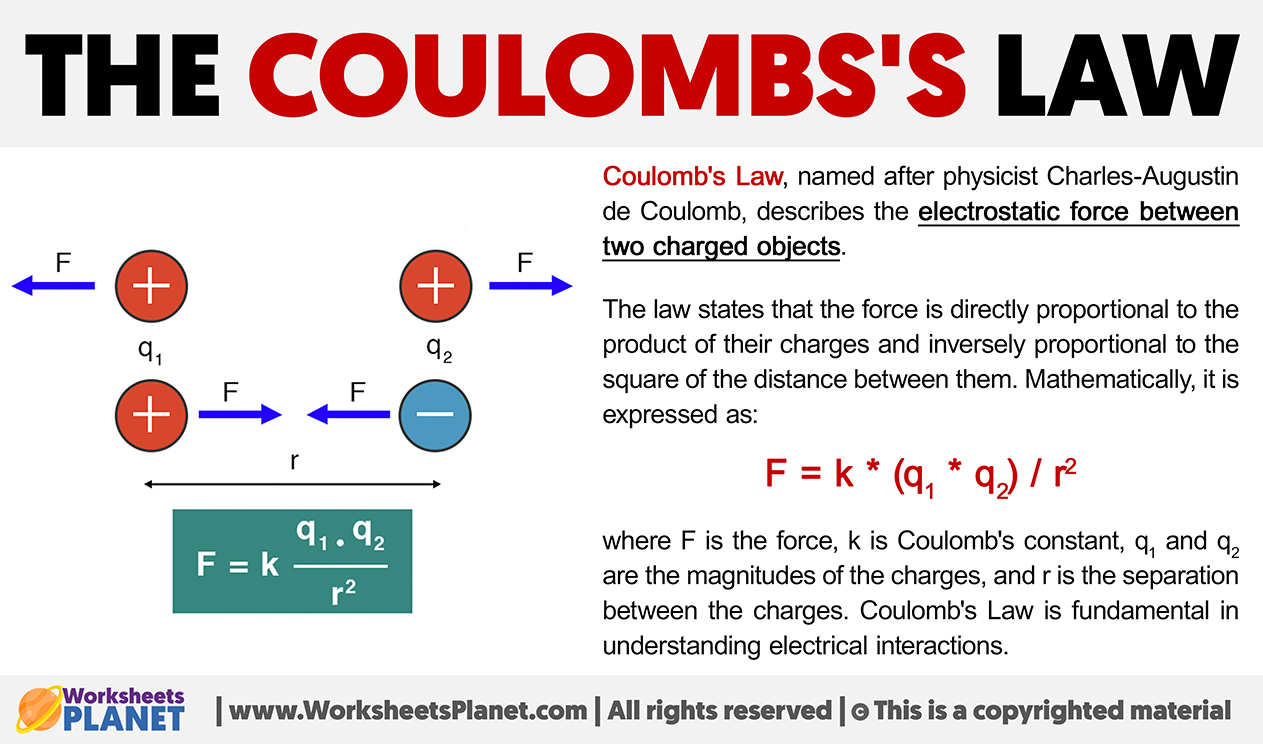
Coulomb’s Law, named after physicist Charles-Augustin de Coulomb, describes the electrostatic force between two charged objects.
The law states that the force is directly proportional to the product of their charges and inversely proportional to the square of the distance between them. Mathematically, it is expressed as:
F = k * (q1 * q2) / r2
where F is the force, k is Coulomb’s constant, q1 and q2 are the magnitudes of the charges, and r is the separation between the charges. Coulomb’s Law is fundamental in understanding electrical interactions.