The Lewis structure is a symbolic representation used in chemistry to illustrate the arrangement of valence electrons in a molecule or ion. Named after Gilbert N.
Lewis, it consists of elemental symbols representing atoms and dots or lines representing valence electrons.
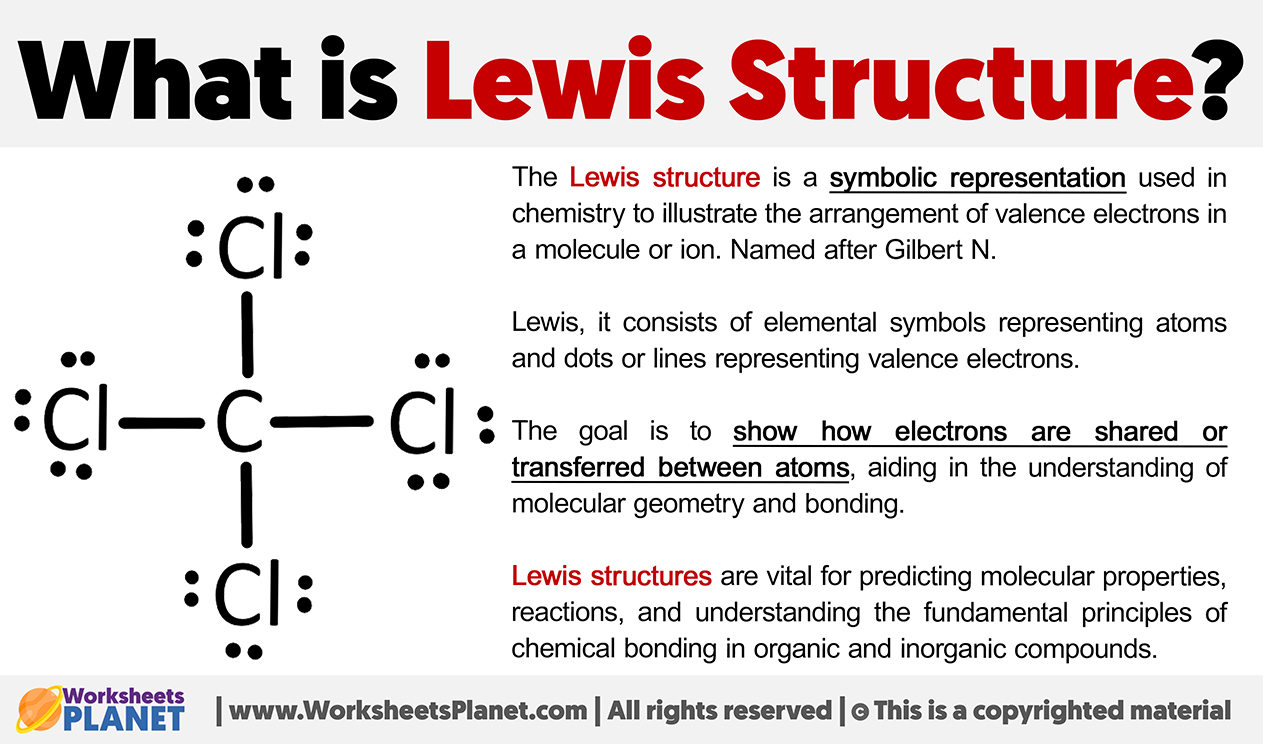
The goal is to show how electrons are shared or transferred between atoms, aiding in the understanding of molecular geometry and bonding.
Lewis structures are vital for predicting molecular properties, reactions, and understanding the fundamental principles of chemical bonding in organic and inorganic compounds.

