A metal element is a type of chemical element characterized by its conductivity of heat and electricity, malleability, ductility, and metallic luster.
Metals are typically found on the left side of the periodic table and include well-known elements such as iron, copper, and gold. They form positive ions in chemical reactions and often exhibit high melting and boiling points.
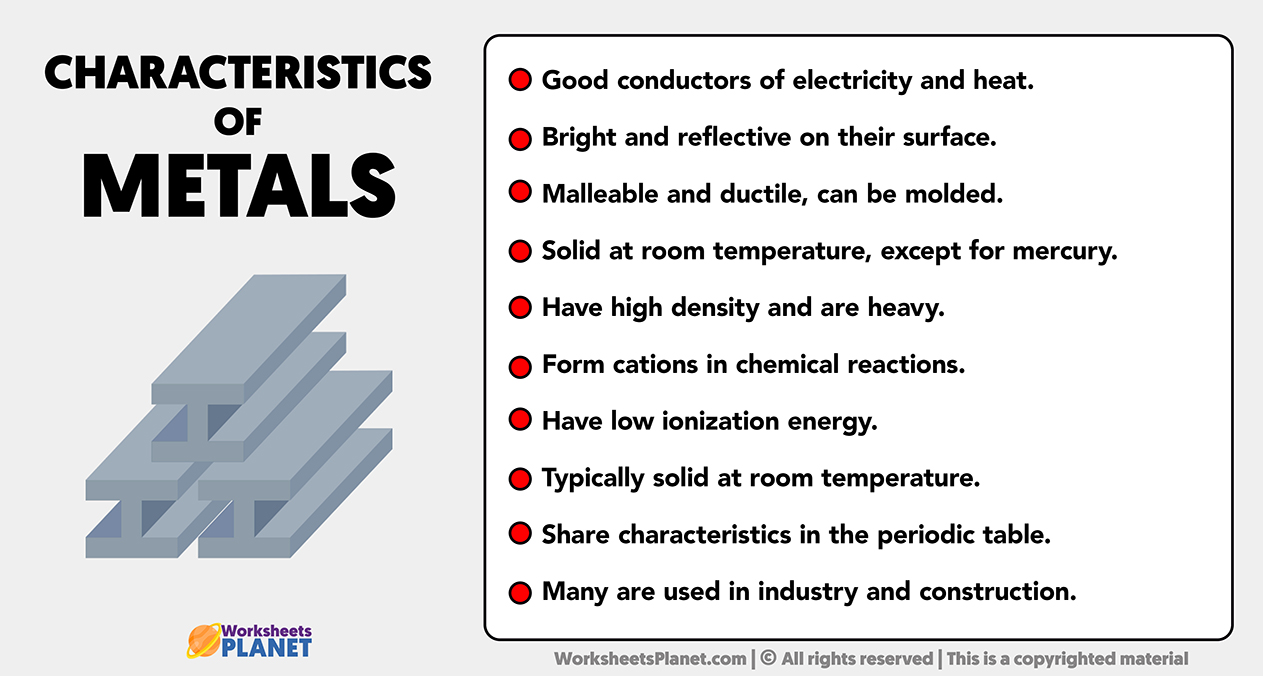
Main Characteristics of Metals
- Good conductors of electricity and heat.
- Bright and reflective on their surface.
- Malleable and ductile, can be molded.
- Solid at room temperature, except for mercury.
- Have high density and are heavy.
- Form cations in chemical reactions.
- Have low ionization energy.
- Typically solid at room temperature.
- Share characteristics in the periodic table.
- Many are used in industry and construction.

