An atom is the basic building block of matter, consisting of a nucleus containing positively charged protons and uncharged neutrons, surrounded by negatively charged electrons.
These subatomic particles determine the atom’s properties. Atoms combine to form molecules, the foundation of all substances. Understanding atomic structure is fundamental to comprehending the nature of elements and the composition of the universe.
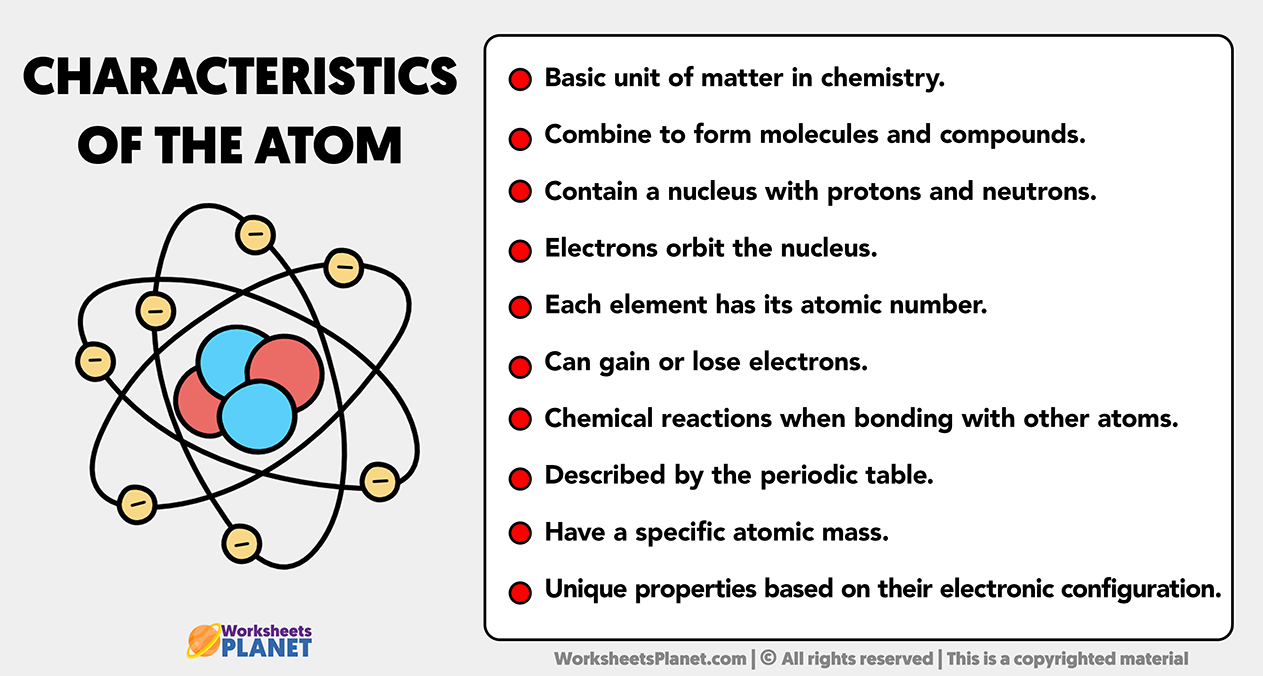
Main characteristics of the atom
- Basic unit of matter in chemistry.
- Combine to form molecules and compounds.
- Contain a nucleus with protons and neutrons.
- Electrons orbit the nucleus.
- Each element has its atomic number.
- Can gain or lose electrons.
- Chemical reactions when bonding with other atoms.
- Described by the periodic table.
- Have a specific atomic mass.
- Unique properties based on their electronic configuration.

